Operating Policies and Procedures Manual 4th Ed
Our Operating Policies and Procedures (OPP) provide guidance on the way Pharmac carries out its statutory role and functions.
On this page
Fourth edition | 27 January 2017
Updated for references to the Pae Ora (Healthy Futures) Act 2022 | August 2022
1. Pharmac
1.1 Our objective and functions
Pharmac is a Crown entity governed by a Board accountable to the Minister of Health[1].
Pharmac's objective is to secure for eligible people in need of pharmaceuticals, the best health outcomes that are reasonably achievable from pharmaceutical treatment and from within the amount of funding provided[2].
In this context, pharmaceutical means a medicine, therapeutic medical device, or related product or related thing[3].
Pharmac’s functions include:
- maintaining and managing the Pharmaceutical Schedule (Schedule)[4]
- managing incidental matters related to the Schedule, including providing subsidies for pharmaceuticals not on the Schedule in exceptional circumstances[5]
- engaging in research to meet our objective[6]
- promoting responsible use of pharmaceuticals[7]
- consulting on matters relating to managing pharmaceutical expenditure[8]
- managing the purchasing of pharmaceuticals including hospital pharmaceuticals on behalf of DHBs[9].
Pharmac must carry out its functions within the amount of funding provided, in accordance with its Statement of Intent and any directions given under the Crown Entities Act 2004[10].
Pharmac makes decisions in accordance with its objective, functions and powers, in light of the Government Rules of Sourcing, and consistent with its public law obligations.
Pharmac works within the New Zealand health system alongside the Ministry of Health (including Medsafe), Te Whatu Ora (Health NZ), Te Aka Whai Ora (Māori Health Authority), and a range of other organisations to deliver on its objective and fulfil its functions.
1.2 Statutory advisory committees
Pharmac has two statutory advisory committees[11]:
- a Pharmacology and Therapeutics Advisory Committee (PTAC) to provide objective advice to PHARMAC in relation to pharmaceuticals; and
- a Consumer Advisory Committee (CAC) to provide input from a consumer or patient point of view.
Pharmac may establish specialist advisory groups to provide specialised, ongoing advice on particular groups of pharmaceuticals[12].
PTAC and advisory committee meeting records and Terms of Reference are published on Pharmac's website. There are some limitations on what is published; these are described in the Terms of Reference.
Pharmac is not limited to seeking advice from its statutory committees and may establish any additional means of obtaining advice, including advisory groups, as appropriate.
1.3 The Treaty of Waitangi/Te Tiriti o Waitangi
We have a commitment to upholding the principles of the Treaty of Waitangi / Te Tiriti o Waitangi[13].
1.4 About our Operating Policies and Procedures
The operating policies and procedures contained in this document are intended to provide guidance on the way in which Pharmac carries out its statutory role and functions. Supporting information about Pharmac’s operations is available on our website however this information does not constitute part of the operating policies and procedures.
1.5 Handling of Information
Pharmac seeks to be transparent in its work and also has obligations under the Official Information Act and Privacy Act to provide access to information. Pharmac does however hold a range of information that is confidential, commercially sensitive, or personal. Pharmac therefore needs to balance the need to respect the confidential or sensitive nature of information it holds, while seeking to achieve transparency and ensuring it complies with its legal obligations.
2. Pharmac’s decisions
We refer to our decisions about whether and how particular pharmaceuticals are publicly-funded, as “funding decisions”.
We consider and assess all funding decisions using the Factors for Consideration. When we do so, we carefully evaluate clinical and other evidence for the benefits and suitability of a proposal, and to identify and understand the people who will be affected by it.
While our main task is to allocate pharmaceutical funding, we consider the benefits and costs across the whole health system now and with a long term focus, including the effects for hospitals and primary care, and consider direct costs to patients as well as to all health sector budgets.
Final funding decisions are made by the Pharmac Board or under delegated authority.
2.1 The Pharmaceutical Schedule
PHARMAC maintains and manages the Pharmaceutical Schedule (Schedule)[14] which reflects Pharmac’s funding decisions and with which DHBs must comply[15].
The Schedule lists the pharmaceuticals publicly-funded in New Zealand, including all medicines which may be given in Te Whatu Ora hospitals, and the pharmaceuticals, including medical devices, used in Te Whatu Ora hospitals and for which Pharmac has negotiated national prices.
As part of managing the Schedule, in accordance with our objectives, we determine eligibility and criteria for listed pharmaceuticals.
2.2 Amending the Schedule
Pharmaceutical suppliers, clinicians, consumers, Te Whatu Ora and any other interested parties may approach Pharmac to suggest possible amendments to the Schedule, using the process described in the relevant funding application guidelines.
Pharmac may amend the Schedule as it considers appropriate, including initiating amendments of its own accord. Possible amendments to the Schedule include (but are not limited to):
- listing new pharmaceuticals
- changing the terms on which a pharmaceutical is listed including;
- changing guidelines or restrictions on prescribing and dispensing
- changing the subsidy levels of pharmaceuticals as a result of Pharmac adopting one of the strategies set out in section 3 or by any other means;
- delisting pharmaceuticals or delisting part or all of a therapeutic group or sub-group;
- changing packaging sizes and brand names;
- changing the indications, formulations, presentations or any other feature of a listed pharmaceutical;
- amending the basis on which pharmaceuticals are classified into therapeutic groups and sub-groups; or
- publishing of information or requirements relating to the implementation of contracts for supply to DHB hospitals.
2.3 Procedure for considering an application for funding
Before seeking to initiate an amendment to the Schedule, the party seeking the amendment should contact Pharmac to discuss the nature of its proposed amendment and establish what the appropriate procedure is in its particular case and what sort of information it needs to provide to Pharmac. Further details about procedures for making submissions may be found in Pharmac’s website.
- The procedure to be followed in respect of an application for an amendment to the Schedule may vary depending on a number of factors, including (but not limited to):
- the nature of the amendment (e.g., new listing, delisting, classification);
- who has initiated the amendment (e.g., Pharmac, PTAC, supplier, clinician, interested parties);
- the type of pharmaceutical being listed (e.g., a new pharmaceutical or a generic pharmaceutical);
- whether the amendment would result from an RFP, tender, listing contract or some other arrangement; or
- whether the amendment is a result of Pharmac adopting a new strategy.
- Pharmac may require a party initiating an amendment to the Schedule to provide in their application relevant information, including (but not limited to):
- pharmacological information (forms, strength, indications, dosages, contra- indications etc);
- therapeutic information (main therapeutic claims, advantages/ disadvantages when compared with other pharmaceuticals etc);
- price information (proposed price, price overseas, other pricing proposals);
- epidemiological information (number of people with the particular condition, number likely to be prescribed the pharmaceutical etc);
- market information (expected sales etc);
- detailed information on the costs and benefits of the pharmaceutical (e.g., reductions in expenditure; improvements in longevity and/or quality of life etc); and
- information regarding packaging and pack sizes.
- Subject to Pharmac's right to prioritise its consideration of proposed amendments, Pharmac is not bound to consider any proposed amendment until the party initiating the amendment has complied with all the conditions set by Pharmac, including (but not limited to):
- providing non-biased information;
- setting out the basis for any estimates or assumptions made;
- providing a synopsis on all material issues; and
- providing comprehensive and detailed cost/benefit information.
The diagram below provides a simplified, indicative guide to the process that Pharmac will usually follow when listing a pharmaceutical on the Schedule. Pharmac is not bound to follow the process set out in the diagram and may vary this process or adopt a different process where appropriate.
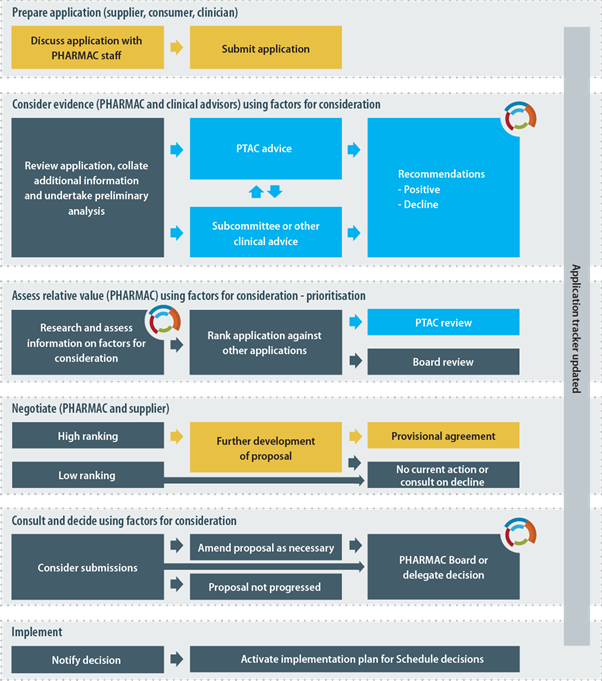
2.4 Special Authorities
Some pharmaceuticals listed in the Schedule are funded for people with specific clinical circumstances, as defined for example in the Special Authority criteria. Most Special Authority applications are processed by the Ministry of Health.
Where a person’s clinical circumstances meet the intent of the Schedule, but not the Special Authority criteria, we may use our discretion to grant a Special Authority waiver.
2.5 Hospital Medicines restriction
Some pharmaceuticals listed in Section H of the Schedule are restricted in certain clinical circumstances, referred to as Hospital Medicine restrictions. We have a Hospital Medicine restriction waiver process for circumstances where a person meets the intent of the Schedule, but not the hospital medicine restrictions.
2.6 Exceptions[16]
Pharmac has an Exceptions Framework that outlines the way we generally consider funding decisions that fall outside the regular decision making process for listing. The Exceptions Framework includes the Named Patient Pharmaceutical Assessment (NPPA) policy.
3. How we secure the best health outcomes from available funding
Pharmac uses the Factors for Consideration to determine whether a proposal or decision helps PHARMAC to achieve its statutory objective of securing for eligible people in need of pharmaceuticals the best health outcomes that are reasonably achievable from pharmaceutical treatment and from within the amount of funding provided[17].
Pharmac may adopt a range of strategies in order to achieve its statutory objective and in pursuit of its functions.
3.1 Factors for Consideration
The Factors for Consideration are taken into consideration to inform funding decisions including funding of pharmaceuticals in exceptional circumstances. The extent to which any one particular factor is relevant, if at all, and the influence of each factor is for Pharmac to determine on each occasion within the context of its legal obligations.
Pharmac considers the Factors for Consideration when making a funding decision to determine whether, at that given point in time and relative to other funding decisions being considered, any decision or proposal helps Pharmac achieve its statutory objective.
Where Pharmac makes decisions that do not involve changes to the Schedule (for example, about promoting the responsible use of medicines), it endeavours to use the Factors for Consideration, to the extent that they are relevant to those decisions.
Specific procurement processes may also have additional prerequisites or evaluation considerations, which would be notified and published by Pharmac accordingly. In addition, processes under the Exceptions Framework such as NPPA may include more detailed requirements.
The Factors for Consideration are supplemented by supporting information about how Pharmac interprets the factors and what they include.
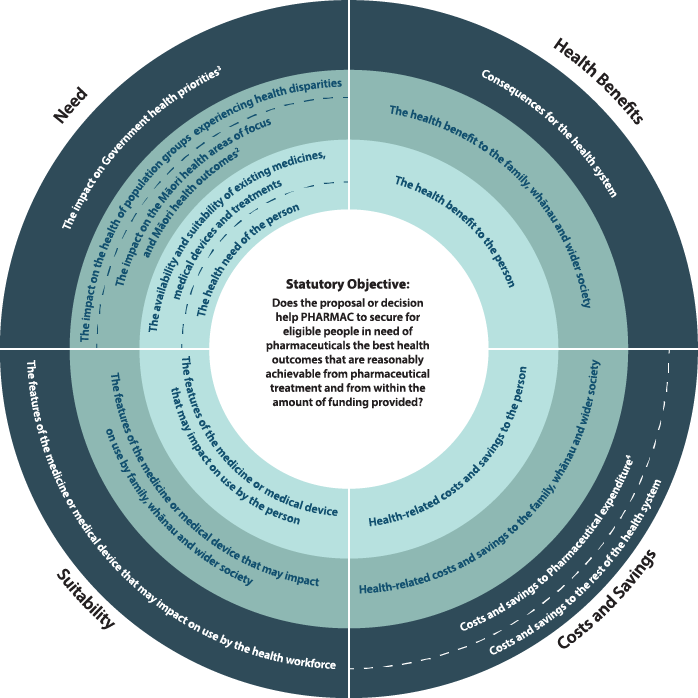
1 The person receiving the medicine or medical device must be an eligible person, as set out in Health and Disability Services Eligibility Direction 2011 under Section 32 of the New Zealand Public Health and Disability Act 2000(external link).
2 The current Māori health areas of focus are set out in Pharmac's Te Whaioranga Strategy
3 Government health priorities are currently communicated by the letter of expectations from the Minister of Health.
4 Includes the impact of the Combined Pharmaceutical Budget (CPB) and/or DHB hospital budgets as appropriate).
If Pharmac considers it appropriate to do so, Pharmac may consult with relevant stakeholders on any proposal, taking this feedback into account before a decision is made. Final decisions are made by the Pharmac Board or its delegate.
3.2 Pharmac’s strategies
Pharmac uses a range of strategies to assist it to achieve its objective.
In addition to standard commercial practices, including competitive processes, specific strategies adopted by Pharmac may include those described below.
Pharmac is not bound to pursue any particular strategy and may modify or depart from a strategy previously adopted, including not applying the strategy the same way in all situations, or adopting new strategies.
When adopting a new strategy, Pharmac will comply with its public law obligations and any decision will be consistent with Pharmac’s statutory objective.
(a) Contracting and contract management
PHARMAC has standard terms of listing for both community and hospital pharmaceuticals. These are updated periodically and available on request.
The majority of pharmaceuticals in the Schedule are listed as a result of listing agreements with the supplier.
A listing agreement details the terms of listing of a product on the Schedule.
Listing agreements may also include special terms relating to particular products, including, but not limited to, rebates and risk sharing arrangements, restrictions on access and protection against de-listing or price reduction.
Any special terms in a given agreement depend on the commercial arrangement we negotiate with the supplier.
(b) Reference pricing
Pharmac sometimes classifies pharmaceuticals into different therapeutic sub-groups. A therapeutic sub-group is a set of pharmaceuticals that, in Pharmac’s opinion, produce the same or similar therapeutic effect in treating the same or similar condition or conditions.
Where reference pricing is applied, all pharmaceuticals within a therapeutic sub-group are subsidised at the level of the lowest-priced pharmaceutical in that sub-group.
(c) Parity pricing
Parity pricing involves reducing the subsidy for a pharmaceutical in a particular therapeutic sub-group to the level of a subsidy for a pharmaceutical in any other sub-group.
(d) Risk sharing
Risk sharing means entering into arrangements involving sharing of financial or other risks between Pharmac and a pharmaceutical supplier, including, but not limited to, rebate arrangements for a particular pharmaceutical or market.
(e) Cross-deal and bundling arrangements
Cross-deal and bundling arrangements involve a decision requiring amendments to the Schedule in respect of more than one pharmaceutical (whether or not those pharmaceuticals are in related therapeutic sub-groups).
(f) Annual tender
Pharmac runs an annual tender process for some pharmaceuticals. Tenders may include contractual terms that reflect that the successful tenderer’s brand of a particular product has sole or other special supply status in the community or Te Whatu Ora hospital market for a period of time. We consult on the annual tender list before it is issued.
3.3 Implementation of funding decisions
Pharmac manages implementation of its funding decisions. We determine an appropriate approach to implementing each funding decision we make. Pharmac liaises with others in the sector with similar responsibilities for implementation (for example, the Ministry of Health in respect to changes to the National Immunisation Schedule). This can include support for consumers, Te Whatu Ora, prescribers, pharmacists and other clinicians, and consideration of distribution and funding arrangements.
3.4 Promoting responsible use of pharmaceuticals
We may undertake a range of activities to support Pharmac’s statutory function to promote the responsible use of pharmaceuticals[18], for example population health activities.
Population health activities are developed in response to evidence-based analysis and identified unmet needs, and aim to improve access and promote responsible use of pharmaceuticals.
Further reading
Government Rules of Procurement(external link)
Principles of Te Tiriti o Waitangi(external link)
Relevant Acts
Crown Entities Act 2004(external link)
Official Information Act 1982(external link)
Privacy Act 2020(external link)
Other agencies
Ministry of Health(external link)
About Pharmac
Making a funding application to amend the Schedule
Make a Special Authority application
Footnotes
[1] Pae Ora (Health Futures) Act 2002 (Pae Ora Act) (section 67)(external link), and Crown Entities Act 2004(external link).
[2] Pae Ora Act, section 68(external link) “(a) to secure for eligible people in need of pharmaceuticals, the best health outcomes that are reasonably achievable from pharmaceutical treatment and from within the amount of funding provided; and (b) any other objectives it is given by or under any enactment, or authorised to perform by the Minister by written notice to the board of Pharmac after consultation with it.”
[3] Pae Ora Act, section 4(external link) “pharmaceutical means a medicine, therapeutic medical device, or related product or related thing.”
[4] Pae Ora Act, section 69(1)(a)(external link) “to maintain and manage a pharmaceutical schedule that applies consistently throughout New Zealand, including determining eligibility and criteria for the provision of subsidies”.
[5] Pae Ora Act, section 69(1)(b)(external link) “to manage incidental matters arising out of paragraph (a), including in exceptional circumstances providing for subsidies for the supply of pharmaceuticals not on the pharmaceutical schedule”.
[6] Pae Ora Act, section 69(1)(c)(external link) “to engage as it sees fit, but within its operational budget, in research to meet the objectives set out in section 68(1)(a)”.
[7] Pae Ora Act, section 69(1)(d)(external link) “to promote the responsible use of pharmaceuticals”.
[8] Pae Ora Act, section 70(external link) “In performing its functions, Pharmac must, when it considers it appropriate to do so, (a) consult on matters that relate to the management of pharmaceutical expenditure with any sections of the public, groups, or individuals that, in the view of Pharmac, may be affected by decisions on those matters; and (b) take measures to inform the public, groups, and individuals of Pharmac’s decisions concerning the pharmaceutical schedule.”
[9] Pursuant to a Ministerial authorisation dated 4 September 2001, under the then section 48(e)(external link) (now section 69(1)(e)(external link)) “any other functions it is for the time being given by or under any enactment, or authorised to perform by the Minister by written notice to the board of Pharmac after consultation with it.”; “Pharmac is authorised to manage the purchasing of any or all pharmaceuticals, whether used either in a hospital or outside it, on behalf of DHBs”. August 2001. DHBs are now Te Whatu Ora.
[11] These committees are established under section 71(1) of the Pae Ora Act(external link). “The board of Pharmac must establish the following advisory committees under clause 14(1)(a) of Schedule 5 of the Crown Entities Act 2004(external link): (a) a pharmacology and therapeutics advisory committee to provide objective advice to Pharmac on pharmaceuticals and their benefits; (b) a consumer advisory committee to provide input from a consumer or patient point of view.”
[12] Members are appointed under clause 14(1)(a) of Schedule 5 of the Crown Entities Act 2004(external link).
[13] To avoid any doubt our statement does not entitle any person to preferential treatment on the basis of race, and does not limit section 73 of the Human Rights Act 1993(external link) (which relates to measures to ensure equality).
[14] Pae Ora Act, section 69(1)(a)(external link) “to maintain and manage a pharmaceutical schedule that applies consistently throughout New Zealand, including determining eligibility and criteria for the provision of subsidies”.
[15] Operational Policy Framework, section 14.4.3(external link): “Each DHB is required to: (a) comply at all times with the rules of the Pharmaceutical Schedule and with any of Pharmac’s decisions related to the Pharmaceutical Schedule…” See also Pae Ora Act, section 14(3) “In performing any of its functions in relation to the supply of pharmaceuticals, Health New Zealand [Te Whatu Ora] must not act inconsistently with the pharmaceutical schedule.”
[16] Pae Ora Act, section 69(1)(b)(external link) “to manage incidental matters arising out of paragraph (a), including in exceptional circumstances providing for subsidies for the supply of pharmaceuticals not on the pharmaceutical schedule”.
[17] Pae Ora Act, section 68(1)(a)(external link) “to secure for eligible people in need of pharmaceuticals, the best health outcomes that are reasonably achievable from pharmaceutical treatment and from within the amount of funding provided.”
[18] Pae Ora Act, section 69(1)(d)(external link) “to promote the responsible use of pharmaceuticals”.


